Harnessing Electricity from Lemons: A Laboratory Experiment, Secondary 3 Science
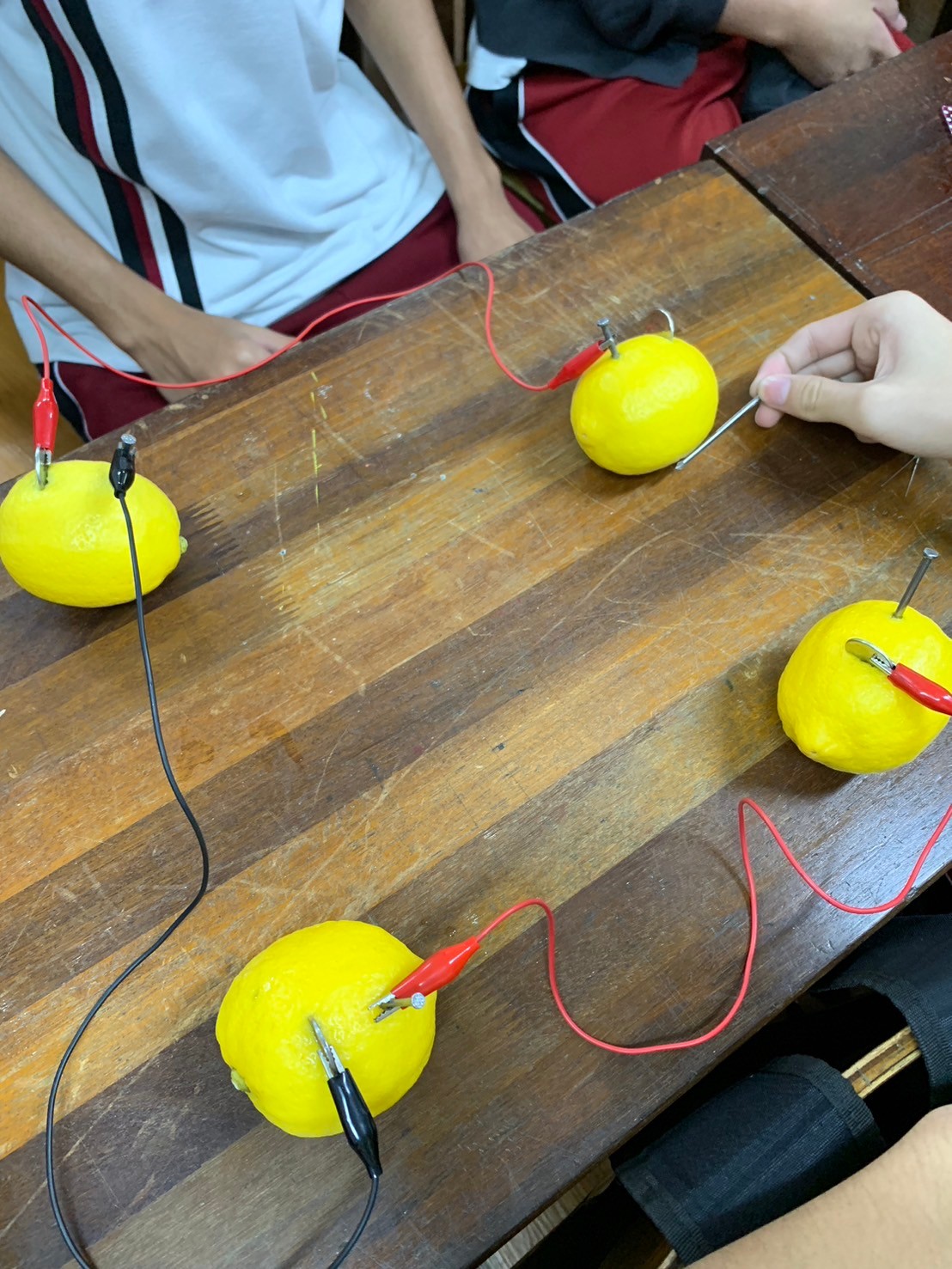
The Lemon Battery experiment is a fascinating demonstration of how chemical energy can be converted into electrical energy. This experiment was conducted by Mathayum 3 students to explore the principles of electrochemistry and prove that electricity can flow through a lemon, generating a small voltage capable of lighting up a bulb. The experiment aimed to demonstrate that an electrolyte, such as the citric acid in a lemon, can facilitate the movement of electrons, thereby producing an electric current.
For this experiment, the students used fresh lemons, copper strips, zinc strips, connecting wires, an LED bulb, and a digital multimeter. The lemons were rolled gently to release juice inside, ensuring better conductivity. A zinc strip and a copper strip were inserted into each lemon without touching each other. The strips in different lemons were then connected using alligator clips, with copper attached to zinc in a series circuit. A digital multimeter was used to measure the voltage generated by a single lemon and by multiple lemons connected together. Finally, the students attempted to light up a small LED bulb by linking several lemon batteries in series to increase the voltage.
The experiment proved that a lemon battery can generate electricity, although the voltage is small and insufficient to power large electrical devices. This provided students with a hands-on understanding of electrochemical reactions and the conversion of chemical energy into electrical energy. The results emphasized the importance of electrolyte solutions in facilitating electron flow and illustrated the basic principles behind batteries used in daily life.